The Cinch Annulizer is developed to provide a percutaneous, quick, safe and simple solution to a complex problem. It allows the tricuspid valve to function as it is intended and leaves a mimimal anatomic footprint. The Cinch Annulizer is designed and tested by experienced structural heart interventionalists and developed with the world-leading engineering team at Resolution medical, MN, US. IP is maintained through Hoffman Eitle, London.
The tech has been developed through 3 years of testing and iterations based on test data and user input including bench at Resolution Medical, beating heart model at Lifetec Group and successful In-vivo deployment in an animal modal at the pre-clinical reference lab at IMMR/Veranex in Paris with extremely encouraging results.
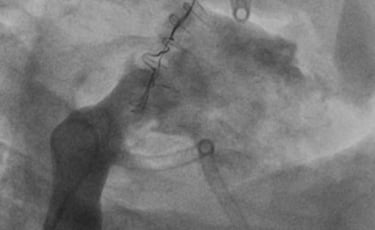
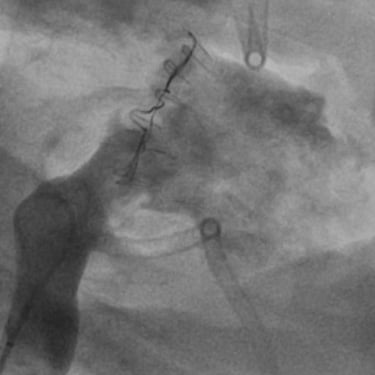
CINCH ANNULOPLASTY DEVICE
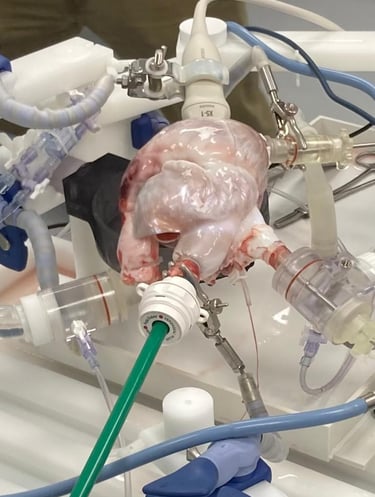
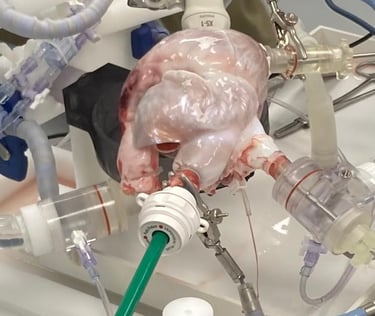
The Cinch Annulizer is currently at a pre-clinical stage and is not approved for human use by any regulatory body.
